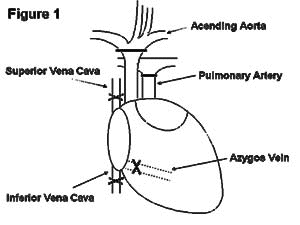
Figure 1
Donor operation
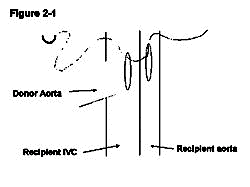
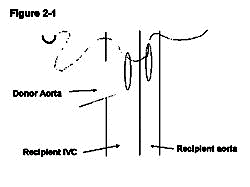
Implantation of the vascularised heterotopic heart graft
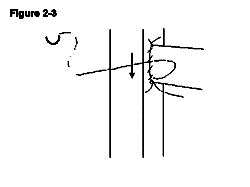
1.1 The method of cardiac transplantation in mice |
1.1.1 Introduction |
|
The mouse heterotopic fully vascularized cardiac transplantation model has come into widespread use since its introduction by Drs. Robert Corry and Paul S. Russell in 1973 (1). This model is of particular value because it allows the fate of fully vascularized organ grafts to be evaluated in a species where a large number of genetically well-characterized strains and newer transgenic and gene knockout animals are available and for which there exists a very large number of immunological reagents.
In this model the donor ascending aorta is anastomosed end-to-side to the recipient abdominal aorta and the donor pulmonary artery is sutured to the recipients inferior vena cava (IVC). Hearts transplanted heterotopically behave functionally as aorto-caval fistulae. Blood enters the donor ascending aorta from the recipient abdominal aorta and is diverted into the coronary arteries by the closed aortic valve. After the myocardium is perfused, venous blood drains into the right atrium through the coronary sinus and is pumped back into the recipients IVC by the right ventricle.
The procedure is carried out using ideally an operating microscope (such as Carl Zeiss OPMI1-FC or OPMI6-CH) at a magnification of between 4 x - 25 x. Operating time is less than one hour and overall operative mortality is less than 5% in experienced hands.
Details of the procedure are as follows:
1.1.2.1 Anaesthesia and Analgesia
1.1.2.2 Recipient preparation
Using two cotton buds the abdominal aorta and IVC are dissected free from the surrounding tissues in the retroperitoneum exposing the lumber arteries and veins which lie directly posterior coming from the great vessels in two or three groups between the renal vessels and the bifurcations inferiorly. The two or three groups of lumber vessels are ligated individually with 7-0 silk ties. If the recipient is male, you find two vessels on the IVC. These vessels are isolated from the IVC. Scoville-Lewis clamp (Downs Surgical Ltd., UK) is positioned at the proximal side of bifurcation interrupting flow in both the aorta and IVC. Another clamp is positioned just below the renal vessels in a similar fashion. Clamping in this order ensures partially filled vessels which will facilitate the aortotomy and venotomy. If you find the IVC contimues to fill after cross clamping, the ligation of lumber vessels shoud be checked. Under ideal conditions cross-clamping for as long as two hours is acceptable.
1.1.2.3 Donor operation
1.1.2.4 Implantation of the vascularised heterotopic heart graft
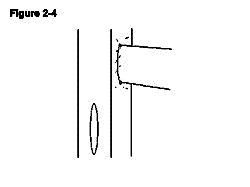
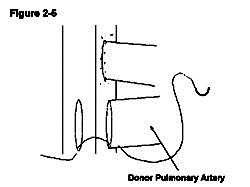
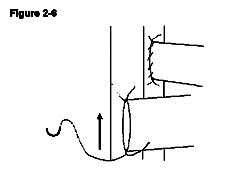
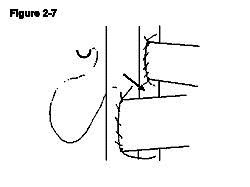
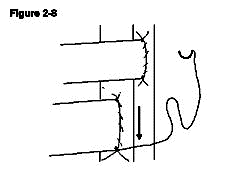
The donor pulmonary artery is now anastomosed to the recipient IVC. A venotomy is made in the same way as the aortotomy made at the level inferior to the aortotomy leaving a space between the two of approximately 3mm, which allows easier access for anastomosing the second side (Figure 2-4). In contrast to the recipient's aorta anastomosis, a stay suture is first placed at the distal apex bringing the donor pulmonary artery and the recipient IVC together. The ends of the stay suture are tied leaving a 5mm length of suture on one end and the needle on the other end (Figure 2-5). Similarly, the proximal apex of the donor pulmonary artery and the recipient IVC are brought together with a10-0 suture. The ends of the proximal stay suture are cut to a length of approximately 2mm and 5mm (Figure 2-6). Stay sutures in this order ensure the same length of both sides of donor pulmonary artery which makes the anastomosis more straightforward. The anastomosis is then completed with the distal stay suture in a clockwise direction with a continuous running stitch. The distance from the distal apex to the proximal apex is covered with four throws (Figure 2-7). The stitch is tied to the proximal stay suture. At this point the needle is passed between the two donor vessels and the donor heart is flipped from the left side of the animal to the right (Figure 2-7). The second side of the anastomosis is completed in a similar manner continuing from the proximal apex to the distal apex and is tied to the distal stay suture. The anastomosis is done similar to the aortic anastomosis except that the distal stay suture is done first and that a continuous running suture goes in clockwise fashion (Figure 2-8).
The distal clamp is removed first to check the anastomosis of the IVC. After appoximate 20 to 30 seconds the vessel will fill and the anastomosis will become competent. The small pieces of moistened haemostatic agent Spongostan (Johnson and Johnson Ltd., UK) are placed around each anastomosis. After ensuring adequate haemostasis the proximal clamp is slowly removed. Normally the donor heart will fill with blood immediately, become bright red in colour, and begin to contract. Provided that there is no further bleeding, the intestines and testes may be returned to the abdominal cavity. The abdominal incision is closed in two layers with 4-0 chromic catgut with needle (Davis and Geck, UK). Separately, the muscle and skin layers are closed with a continuous running stitch.
1.1.2.5 Recovery |
1.1.3 Additional usuful points |
| (1) 4 or 5 stitches on each side excluding stay sutures are enough and sufficient. The next and last stitches of continuous running suture between donor and recipient aorta should be placed with a short bite and close to the stay suture to avoid stenosis of recipient aorta. (2) Two stay sutures must be located on the opposite side. If the distal stay suture on IVC is first performed, you can recognise easily where the proximal stay suture should be. (3) Some space between proximal end of venotomy and distal end of aortotomy should be prepared. Otherwise, the second side of IVC anastomosis can not be performed safely and confidently. (4) The best length of venotomy and aortotomy is the same as the opening of pulmonary artery and ascending aorta of donor. However, smaller is much better than larger. (5) Loose sutures are better than over-tight ones. It is much easier to control a small amount of bleeding than to attempt to open an occluded vessed. |
1.1.4 Trouble shooting |
| (1) After release the distal clamp, check the bleeding from the anastomosis of recipient IVC and donor pulmonary artery. If the bleeding continues as the IVC fills, it can be controlled with haemostatic sponge. Defects in suturing may be repaired at this point. (2) After releasing the proximal clamp, there is usually some bleeding from the anastomosis of the recipient and donor aorta. This usually resolves spontaneously. If bleeding continues, the proximal clamp can be replaced and the sutureing defect repaired. (3) If the bleeding is from the atrium, it can be ligated at the base with a 7-0 silk tie. (4) If you find that the bleeding is from a coronary vessel, haemastatic sponge can be applied. This is sometimes successful. (5) If blood loss is severe, 1ml of warm saline shoulc be given intraperinearlly or subcutaneously. (6) If you find both lower legs paralyzed after operation, there is no chance of survival because of occlusion of the abdominal aorta at the anastomosis. If you find one lower leg paralyzed, there is some chance of survival as this might be due to the air or fat embolism. |
1.1.5 Assessment of graft survival |
| The function of the transplanted hearts is followed by abdominal palpation . Absence of a palpable heart beat is considered as rejection. This is normally easy to determine. However, in some cases the decision can be more difficult. In this case electrocardiogram (2) or direct visualisation of the graft is invaluable. |
1.1.6 2nd cardiac allografts into the abdomen or neck |
| Replacement of the primary graft with a second heart is possible (3). A second heart can also be transplanted into the recipient's neck. Briefly, the donor ascending aorta is anastomosed end-to-side to the recipient carotid artery and the donor pulmonary artery is sutured end-to-side to the recipient external jugular vein. If a cuff technique is availabe, both anastomoses can be completed using 22 and 24 gauge cuffs for recipient vein and artery, respectively. This approach saves time and increases the success rate (4). |
1.1.7 Non-vascularized cardiac allografts into the ear |
| Prior to the introduction of the vascularized cardiac graft model in the mouse, neonatal non-vascularized cardiac grafts were transplanted into the ear using the method originally described by Fulmer et al (5). Briefly, the heart is excised from newborn mice and implanted into the dorsal base of the pinna of the ear. The implant is subcutaneous and surgical incision is gently closed. The majority of grafts in normal syngeneic mice start to present contractile activity 7 to 10 days after transplantation. |
1.2 The results of cardiac allografts in mice |
1.2.1 MHC disparities |
| The rejection of heart grafts transplanted between MHC disparate mice is shown in Table 1. Usually allogeneic cardiac grafts in naive mice are rejected in approximately 7 to 12 days. It is clear that both MHC and non-MHC factors can play a role in the rejection of MHC incompatible heart grafts (6). Congenic strains have therefore been extremely useful in attempts to determine the relative importance of these factors in allograft rejection. It is important to note that in contrast to the mouse skin transplant model, time taken to reject a heart grafts is unpredictable. For example, in a small number of strain combinations, unmodified recipients accept MHC mismatched heart grafts indefinitely(6-8). |
1.2.2 Limited MHC disparities |
| The survival of murine heart grafts transplanted across limited MHC barriers is shown in Table 2. Hearts transplanted across isolated class II incompatibilities (IA/IE) are usually rejected but have been reported to survive for more than 50 days in only 1 donor recipient combination(Table 2, groups 12-14). In contrast, hearts transplanted across isolated class I MHC barriers(K or D) survived for greater than 50 days in 7 of the 10 donor recipient combination tested(Table 2, groups 1-10). This suggests that in the mouse class II is the dominant MHC antigen barrier in unmodified recipients. |
1.2.3 Non-MHC disparities |
| Initially it was thought that although transplantation across multiple non-MHC incompatibilities would result in the rejection of a primarily vascularized cardiac allograft, the rejection response elicited by multiple non-MHC antigens would not be as powerful as that evoked by a full MHC mismatch(12,13). However, it has been shown that multiple non-MHC disparities can induce acute rejection, both of tissue grafts(6) and vascularized heart grafts(6,10,14) and that the tempo of rejection is similar to that induced by full MHC incompatibilities (Table 3). |
1.2.4 Transgenic and knockout or gene deleted mice |
| One of the advantages for using the mouse model is the availability of many types of knockout and transgenic animals. Campos et al.(16) indicated the importance of MHC class II locus products in the rejection of cardiac allografts by using MHC class I, MHC class II and both class I and II deficient mice(Table 4). Qian et al.(17) reported the importance of MHC class I in initiating second-set allograft rejection again using mice deficient in MHC class I and class II genes. A recent study by Krieger et al.(18) examined the survival of adult heart grafts in CD4, and CD8 knockout recipients. The data obtained indicated that CD4+ but not CD8+ T cells are required to initiate allograft rejection, supporting data from earlier studies(19,20). Cytokine transgenic and knockout mice have also used on cardiac transplantation models(21-23). |
Figure 1  |
|
| Figure 2 Implantation of the vascularised heterotopic heart graft |
|
 |
 |
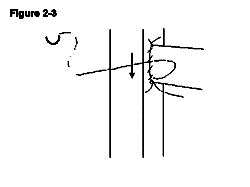 |
 |
 |
 |
 |
 |
| References |
| 1. Corry RJ et al. (1973) Primarily vascularized allografts of hearts in mice: the role of H-2D, H-2K and non H-2 antigens in rejection. Transplantation 16: 343-350 2. Superina RA et al. (1986) Assessment of primarily vascularized cardiac allografts in mice. Transplantation 42: 226-227 3. Pearson TC et al. (1992) Successful secondary heterotopic cardiac transplantation in the mouse. Transplantation 53: 701-703 4. Matsuura A et al. (1991) Simplified mouse cervical heart transplantation using a cuff technique. Transplantation 51: 896-898 5. Fulmer R, AT C, RA L , AG L. Transplantation of cardiac tissue into the mouse ear. Am J Anat 1963; 113: 273 6. Peugh WN et al. (1986) The role of H-2 and non-H-2 antigens and genes in the rejection of murine cardiac allografts. Immunogenetics 23: 30-37 7. Stepkowski SM et al. (1987) The role of class I and class II MHC antigens in the rejection of vascularized heart allografts in mice. Transplantation 44: 753-759 8. Wong W et al. (1996) Syngeneic bone marrow expressing a single donor class I MHC molecule can induce tolerance to a fully allogeneic cardiac allograft. Transplantation 62: 1462-1468 9. Shelby J (1986) Survival times of mouse heart allografts. Mouse Microsurgery Newsletter 1: 2 10. Burdick JF, Clow LW (1986) Rejection of murine cardiac allografts. I. relative role of major and minor antigens. Transplantation 42: 67-72 11. Heelan B (1995) T cell receptor Vb usage in allorecognition: a study of mutant MHC class I antigen mismatch. DPhil Thesis, University of Oxford, Oxford, pp 106-135 12. Barker CF, Billingham RE (1971) Histocompatibility requirements of heart and skin grafts in rats. Transplantation Proceedings 3: 172 13. Bildsoe P (1972) Organ transplantation in the rat. Acta Pathology Microbiology Immunology Scand Section C 80: 221 14. Katz SM et al. (1983) The relative roles of MHC and non-MHC genes in heart and skin allograft survival. Transplantation 36: 96 15. Niimi M et al. (1997) B7-2-+ low density APCs are as effective as B7-2-- small resting B cells in inducing specific unresponsiveness to minor histocompativility antigen in vivo. Dendritic Cells in Fundermental and Clinical Immunology 3: 265-269 16. Campos L et al. (1995) Survival of MHC-deficient mouse heterotopic cardiac allografts. Transplantation 59: 187-191 17. Qian S et al. (1996) Impact of donor MHC class I or class II antigen deficiency on first- and second-set rejection of mouse heart or liver allografts. Immunology 88: 124-129 18. Krieger NR et al. (1996) CD4+ but not CD8+ cells are essential for allorejection. J. Exp. Med. 184: 2013-2018 19. Loveland BE et al. (1981) Cells mediating graft rejection in the mouse. I. Lyt-1 cells mediate skin graft rejection. J. Exp. Med. 153: 1044-1057. 20. Dallman MJ et al. (1982) The role of host and donor cells in the rejection of skin allografts by T cell-derived rats injected with syngeneic T cells. Eur. J. Immunol 12: 511-518 21. Saleem S et al. (1996) Acute rejection of vascularized heart allografts in the absence of IFNg. Transplantation 62: 1908-1911 22. Piccotti JR et al. (1996) IL12 antagonism induces T helper 2 responses, yet exacerbates cardiac allograft rejection. Evidence against a dominant protective role for T helper 2 cytokines in alloimmunity. J Immunol 157: 1951-1957 23. Takeuchi T et al. (1997) Murine interleukin 4 transgenic heart allograft survival prolonged with down-regulation of the Th1 cytokine mRNA in grafts. Transplantation 64: 152-157 |
|
|
|
Copyright (C)2007 Masanori Niimi All rights reserved. |
| ×閉じる |